ʟ-Asparaginases and Their Potential in Biotechnology and Medicine
DOI:
https://doi.org/10.54779/chl20230508Keywords:
ʟ-asparaginase, acute lymphoblastic leukemia, acrylamide, biosensor, biological anti-cancer treatmentAbstract
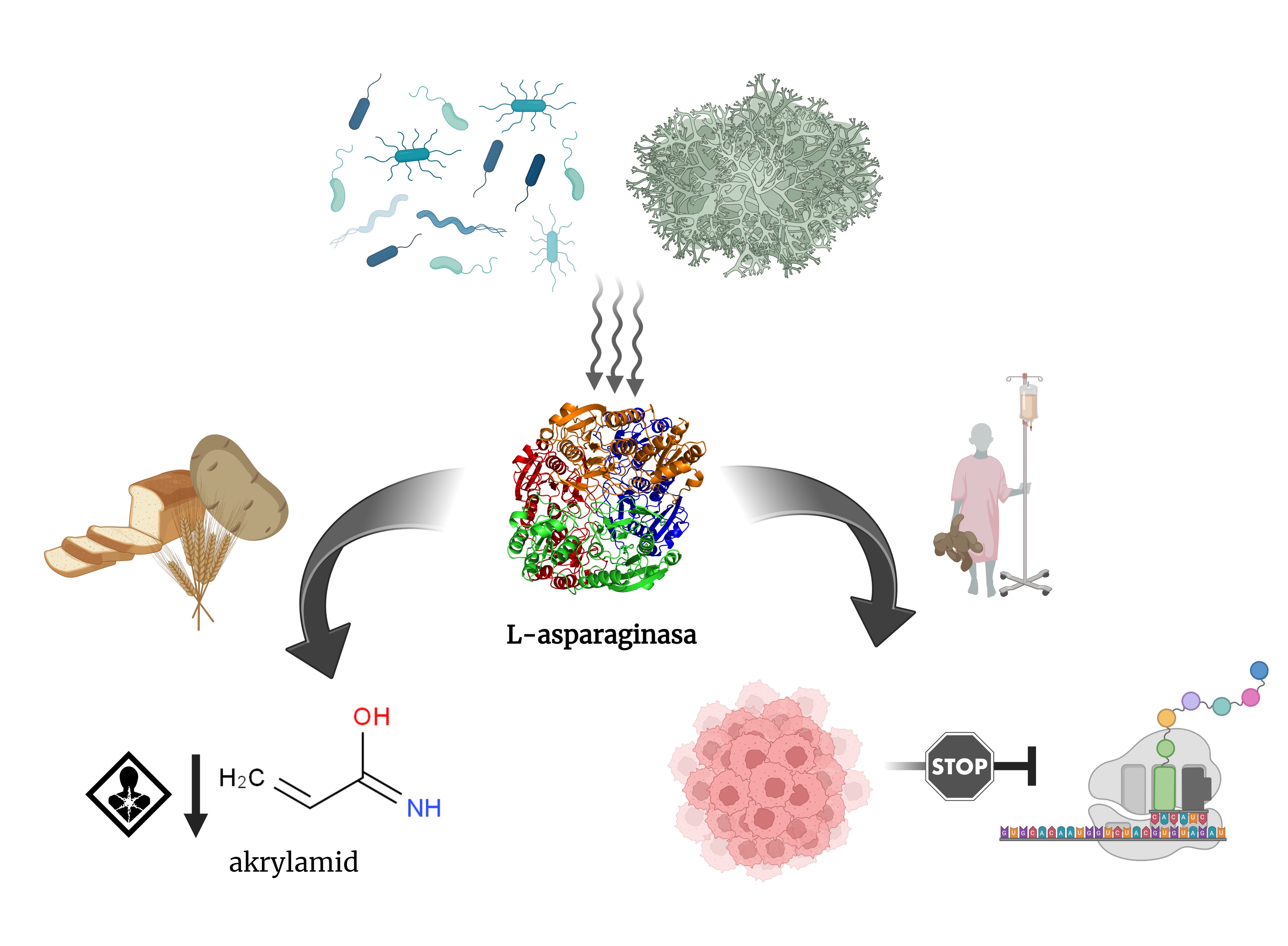
ʟ-Asparaginase (EC 3.5.1.1) is a key enzyme that hydrolyzes ʟ-asparagine to ʟ-aspartic acid and ammonia. This feature of ʟ-asparaginase is used in anti‑cancer therapy to inhibit protein synthesis in cancer cells. Therefore, ʟ-asparaginase is used as a basis for chemotherapy to treat patients with acute lymphoblastic leukemia in pediatrics. Commercial ʟ-asparaginases for healthcare applications are mainly obtained from Escherichia coli and Erwinia chrysanthemi (renamed to Dickeya dadantii). However, the high prevalence of adverse effects complicates the long‑term clinical use of ʟ-asparaginase, and therefore current research focuses on the search for new enzymes or on modifying the properties of enzymes already known. At the same time, ʟ-asparaginase has become indispensable for the food industry in recent years, when it had been recognized as one of the possible tools for removing ʟ-asparagine from foods that are at risk of acrylamide formation during thermal processing. This review provides an overview of the current use of ʟ-asparaginase and its pitfalls.
Full text English translation is available in the on-line version.