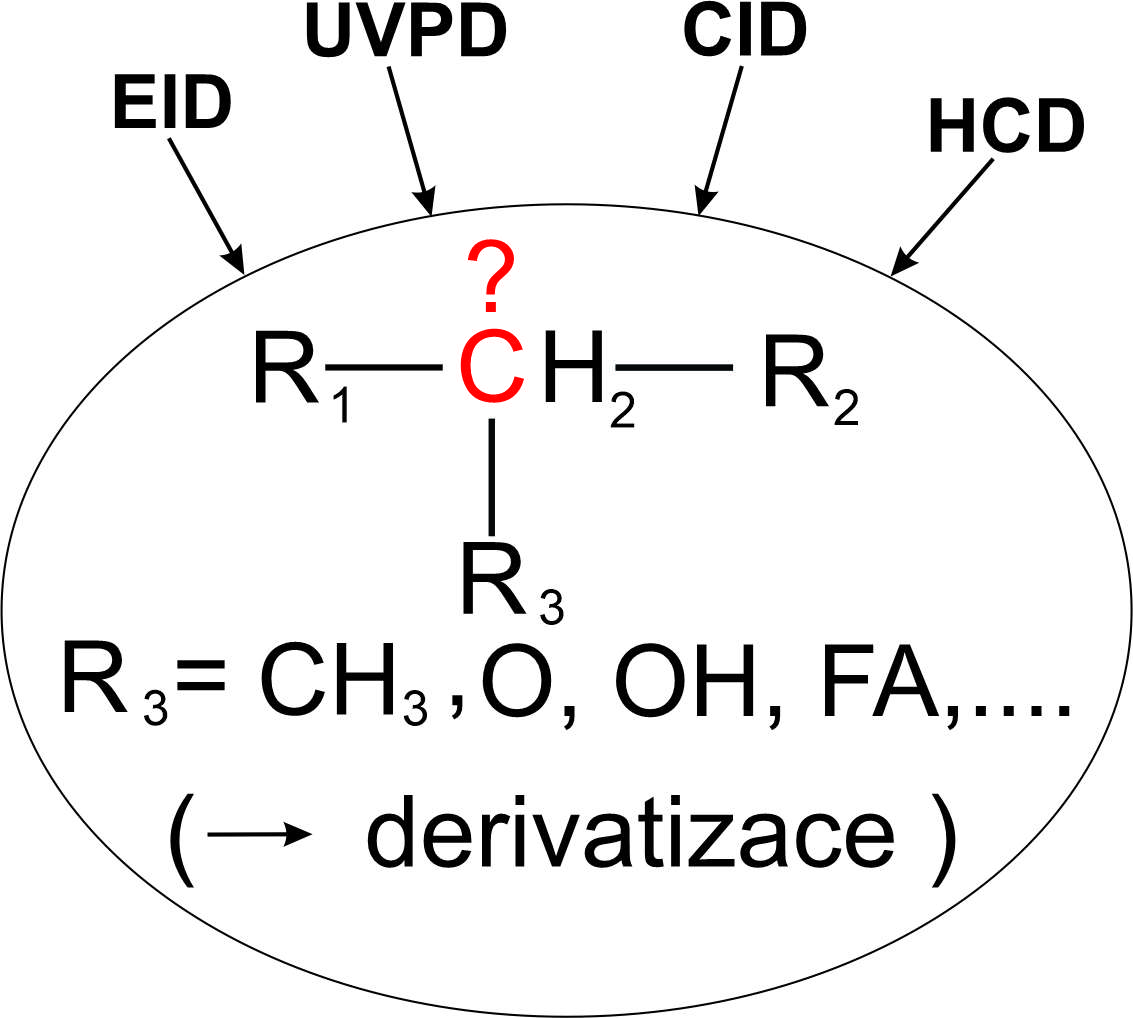
Locations of Functional Groups in the Aliphatic Chains of Lipids and the Arrangement of Aliphatic Chains within Acylglycerolipids by the Methods of Mass Spectrometry
This article is dedicated to Professor Jiří Barek on the occasion of his 75ᵗʰ birthday
DOI:
https://doi.org/10.54779/chl20240594Keywords:
fragmentation, functional groups, carbocyclic compounds, lipids, methyl branching, sn-positions, structural analysisAbstract
Lipids play key roles in many biological processes. Most lipids contain long aliphatic chains whose variable structure contributes to diverse physicochemical properties and biological functions of these molecules. The aliphatic chains of lipids can be straight or branched, with double or triple bonds, or with various functional groups. In recent years, mass spectrometry has made significant progress in the structural analysis of lipids. New methods are based on unconventional fragmentation techniques and new derivatization reactions. They can be used to determine the detailed structure of aliphatic chains, including the position of multiple bonds, functional groups, and the arrangement of aliphatic chains in complex lipids. This review article is a follow-up to two previous articles that dealt with the determination of double bond positions (Chem. Listy 117, 684 (2023), Chem. Listy 117, 747 (2023)). Here, we focus on determining the position of methyl branching, oxygen-containing functional groups, carbocyclic structures, and the stereospecific position of the acyl chain on glycerol.